|
管理员

|
诱导多能干细胞首次被批准用于人体心脏修复!日本科学家将在今年进行实验
诱导性多能干细胞(Induced pluripotent stem cell,下文简称 iPS 细胞),又称人工诱导多能干细胞,是一种由哺乳动物成体细胞经转入转录因子等手段脱分化形成的多能干细胞。2006 年首次由日本科学家山中伸弥团队发现,其本人也因此项技术于 2012 年获诺贝尔生理医学奖。 iPS 细胞一经发现,便引起整个学术界的轰动,人们为找到“重编程”生命的“配方”而欢呼雀跃,诱导多能干细胞的实现,被人们当做是现代医学革命的信号,而今经过了 10 余年的努力,日本科学家在 iPS 细胞的应用路上再进一步。5 月 16 日,日本卫生部为 iPS 细胞的临床应用大开绿灯,批准将其用于心脏衰竭的临床试验。 
At a press conference in Tokyo, cardiac surgeon Yoshiki Sawa announces plans to use tissue derived from induced pluripotent stem cells to treat heart disease.The Asahi Shimbun via Getty Images 图 | Yoshiki Sawa 在东京的新闻发布会上介绍此项临床试验 来自大阪大学(Osaka University)的心脏外科医生 Yoshiki Sawa 将带领团队开展此项临床试验。手术中,医生将向患者心脏表层植入一层人工培育生成的心肌细胞(约 0.1 毫米),植入的细胞可以通过分泌蛋白质等物质来帮助血管生长和心脏功能改善。该临床试验将于明年 3 月开始,初期将有 3 名患者接受治疗,随后将扩展为 10 人左右。如果试验成功,日本将根据其关于再生医学的“快速通道系统”将该临床技术直接商业化。 “全世界都将密切关注(此次临床试验的结果),许多研究团队也正在相同的研究方向上努力,”来自德国汉堡大学(University of Hamburg)的药理学家,同时也是德国心血管研究中心主席的 Thomas Eschenhagen 对此项研究结果充满期待。 相对于临床试验一片看好,学术界对于商业化的态度却忧心忡忡,对于日本 2014 年才推出的这个“快速通道系统”,初衷是加速挽救生命,但在商业化之前并没有充分的数据证明治疗效果是存在缺陷的。很多学者也认为不经长期、对照验证的疗法贸然市场化略显“疯狂”,无法接受。 修补受伤的心
心脏衰竭通常具有呼吸困难、疲劳等症状,尤其在运动、平躺及夜间睡眠时症状加剧。目前心脏衰竭是常见的、高医疗支出且可能致命的疾病。在我国,心脏衰竭患者约有 1000 万,其中约 50% 患者在诊断 5 年后死亡。 目前常见的治疗手段为药物治疗,严重情况下,患者需要依靠辅助性的人工设备或进行心脏移植,但这两种方法都具有局限性:人工设备容易引起并发症,而心脏移植常面临供体短缺的问题。而随着 iPS 细胞技术的实现,患者自身心脏“再生”成为可能。 
图 | 心脏衰竭症状 在生物发育过程中,一个干细胞既可以成为皮肤细胞、心脏细胞,也可以成为神经细胞、肌肉细胞,而引导这个方向的过程就是分化。干细胞在分裂的时候,其子细胞的基因表达受到修饰调控,如 DNA 的甲基化,使其向特定的方向分化,形成了不再具有多能性的体细胞,在整个机体中发挥特定功能。 干细胞就像生命体内的种子,既然器官是从干细胞发育而来,那如果可以获取干细胞,人类自然可以“种器官”。但获取干细胞并非易事,不仅数量少难以辨识,而且获取的过程很可能会对患者产生附加伤害。 虽然生物体每个细胞功能不同,但都有着相同的遗传信息。既然所有的细胞都是同宗同族,那是否有办法让生命“开倒车”,回到最初的状态呢?基于这一想法,2006 年日本科学家山中伸弥首次采用慢病毒载体将 Oct4、Sox2、c-Myc、Klf4 四种转录因子基因转入成体细胞将其“重编程”,转化为类似于胚胎干细胞的多能干细胞。 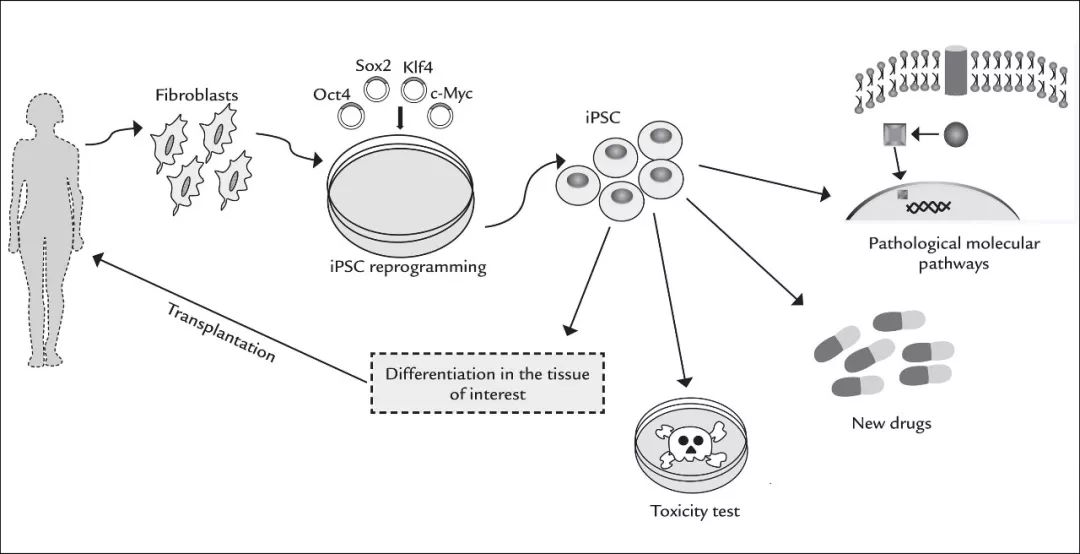
图 | iPS 细胞的原理及应用 iPS 细胞与胚胎干细胞拥有相似的再生能力,理论上可以分化为成体的所有器官、组织。而相比胚胎干细胞,iPS 细胞面临的伦理道德争议较小,且应用该技术可以产生基因型与移植受体完全相同的干细胞,规避了排异反应的风险,因而 iPS 细胞在一定程度上冲击了胚胎干细胞在再生医学中的地位,被认为在再生医学及组织工程方面拥有更为广阔的应用前景。 令人不安的“超前”
用患者轻松可得的皮肤细胞就能“重编程”获取珍贵的诱导多能干细胞,再将其诱导培养成为心肌细胞完成移植,这一生命“魔法”正是 Sawa 团队希望展示的。在临床试验中,研究者将会把一层(厚约 0.1 毫米、长约 4 厘米)含有约 1 亿个心肌细胞的片状结构植入患者心脏表层,帮助患者提升器官功能。从之前的动物实验结果来看,后植入的细胞并没有和之前的心脏组织融为一体,Sawa 认为新植入的心肌细胞的功能是释放生长因子,帮助受损的心肌细胞再生。 这种层状结构的优点是可以使用自身的细胞基质保持结构,无需外来材料。“这种方式既简练又聪明,”Philippe Menasché,这位来自巴黎乔治蓬皮杜欧洲医院(Georges Pompidou European Hospital)的同行表示十分认可。 Sawa 最初的 3 例临床试验一旦开始,随后将会有另 7-10 名患者被招募接受临床试验。一旦临床试验被证明安全,或是有有效的迹象,该项技术将在加速系统下被批准进行商业销售。这一加速系统使研究人员绕过了繁琐而昂贵的大规模临床试验,仅通过小规模的先导试验证明该疗法的安全性和功效。 
但一些研究者对此提出了质疑:批准用于商业化的疗法标准是否太低。即使 iPS 细胞是安全的,但任何手术都可能会有风险,患者很有可能会因为这个安全性和疗效都不那么确定的疗法而放弃其他的治疗。同时,对于这种疗法可能带来的免疫排斥反应及手术本身的风险,都需要患者自身承担。伦理学家和监管机构表示,对于任何疗法来说,它的益处都必须超过风险才有存在的价值。 除了安全评估,一些研究者揪住了这次临床试验的“硬伤”:缺少随机对照试验。“虽然 iPS 细胞疗法充满潜力,”来自京都大学的心脏病学家 Yoshiki Yui 说到,但由于缺乏对照实验,“我们并没有办法知道它是不是真的奏效,”Yui 毫不留情,直指要害,“最大的问题是,目前在日本并没有匹配的评估体系。” 
而卫生部发言人却不以为然,他们认为目前的批准标准已经足够,因为即使被批准商业化应用,研究者仍旧需要持续提供该疗法有效的证据。 对于目前的争论,Sawa 认同对照组对于证明疗效的重要性,但他也指出,目前他仍旧在日本规则的框架下行事—在商业化之前,他可以不采用对照组实验。卫生部的批准只是证明该疗法“科学合理、符合道德伦理的”,而至于该疗法是否真的奏效,“我们会在现在搞清楚”。 -End- 参考: ‘Reprogrammed’ stem cells approved to mend human hearts for the first time The latest clinical use of induced pluripotent stem cells excites researchers, but some fear the therapy will be rushed to market. Scientists in Japan now have permission to treat people who have heart disease with cells produced by a revolutionary reprogramming technique. The study is only the second clinical application of induced pluripotent stem (iPS) cells. These are created by inducing the cells of body tissues such as skin and blood to revert to an embryonic-like state, from which they can develop into other cell types. On 16 May, Japan’s health ministry gave doctors the green light to take wafer-thin sheets of tissue derived from iPS cells and graft them onto diseased human hearts. The team, led by cardiac surgeon Yoshiki Sawa at Osaka University, says that the tissue sheets can help to regenerate the organ’s muscle when it becomes damaged, a symptom of heart disease that can be caused by a build-up of plaque or by a heart attack. “It will excite worldwide attention, as many groups are working in the same direction,” says Thomas Eschenhagen, a pharmacologist at the University of Hamburg in Germany and chair of the German Centre for Cardiovascular Research. The treatment will initially be given to three people over the next year. The team will then seek approval to conduct a clinical trial in around ten patients. If it proves safe, the treatment could then be sold commercially under Japan’s fast-track system for regenerative medicine. The system, introduced in 2014, aims to speed the availability of potentially life-saving procedures. But critics say the system is flawed because it allows treatments to be sold to patients before sufficient data have been collected showing that the procedures work. Mending broken hearts In their technique, Sawa and his colleagues use iPS cells to create a sheet of 100 million heart-muscle cells. From studies in pigs, the team has shown that grafting these sheets of cells — each 0.1 millimetres thick and 4 centimetres long — onto a heart can improve the organ’s function. Sawa says that the cells do not seem to integrate into the heart tissue. He thinks that instead they release growth factors that help to regenerate the damaged muscle. Scientists say one advantage of the sheets is that they create their own cellular matrix, and can maintain their structure without the need for scaffolding made from foreign materials, a feature of some other engineered tissues. “It is a very elegant and clever way to deliver cells,” says Philippe Menasché, a cardiac surgeon at the Georges Pompidou European Hospital in Paris, who has also experimented with making tissue sheets. Pharmacologist Wolfram-Hubertus Zimmermann at the University Medical Centre Göttingen in Germany, who is also developing an iPS treatment for heart disease, says that the latest trial is based on work conducted by Sawa and other colleagues in Japan over the past 15 years. Once Sawa’s team has treated its three patients, it will apply to conduct a clinical trial involving a further seven to ten people. If the treatment proves to be safe, and shows some signs of working, it can be approved for sale under the accelerated system. This allows researchers to bypass expensive large-scale clinical trials aimed at proving efficacy, and instead to use small pilot trials to show that the therapy is safe and demonstrates an indication of efficacy. Insufficient oversight But some researchers say the bar for approving therapies for commercial use is too low. Even if the cells are found to be safe, there are risks associated with any surgery, and patients could give up other therapies for a treatment that might not work. Ethicists and regulators say that the benefits of any new therapy must outweigh the risks. Yoshiki Yui, a cardiologist at Japan’s Kyoto University, says that, as well as meeting the requirements for safety, researchers should show that their treatment is effective, which would require testing it in larger numbers of people than are currently required. The evaluation process should also use randomized, controlled clinical trials, the gold standard for demonstrating efficacy in medical research, he says. The iPS-cell therapy has potential, Yui adds, but under the current approval system, “we won’t know if it works or not” because it won't have been tested in a controlled trial. “The biggest problem is there’s no adequate system of evaluation in Japan,” says Yui. A spokesperson for the health ministry told Nature that the current approval system is sufficient because researchers must still show that a treatment works even if it has been approved for commercial use. Sawa agrees that a control group is important for proving efficacy, but notes that he is abiding by Japan’s rules, which don’t require this before a treatment is made commercially available. He says the health ministry’s approval is an acknowledgement that the therapy “is scientifically and ethically justified” to be tested in patients. “Whether it really works, [we] have to find out now,” he adds. Nature 557, 619-620 (2018) doi: 10.1038/d41586-018-05278-8 https://www.nature.com/articles/d41586-018-05278-8 Stem-cell tests must show success Japan needs to demonstrate that a promising therapy for damaged hearts works as claimed.  Researchers in Japan, led by cardiac surgeon Yoshiki Sawa (second from right) at Osaka University(大阪大学), are pushing ahead with a stem-cell therapy for damaged hearts.Credit: Asahi Shimbun/Getty Researchers in Japan, led by cardiac surgeon Yoshiki Sawa (second from right) at Osaka University(大阪大学), are pushing ahead with a stem-cell therapy for damaged hearts.Credit: Asahi Shimbun/Getty
https://www.nature.com/articles/d41586-018-05284-w 2018-05-30 18:20程序设计/操作系统/技术 诱导多能干细胞首次被批准用于人体心脏修复!日本科学家将在今年进行实验 http://www.sohu.com/a/233474459_354973 |
|
管理员

|
沙发#
发布于:2018-06-19 06:14
Stem-cell tests must show success
EDITORIAL 29 MAY 2018
Stem-cell tests must show success Japan needs to demonstrate that a promising therapy for damaged hearts works as claimed. As we report in a News story this week, Japan is set to push ahead with a promising treatment for heart disease that relies on stem cells. It could soon be made available under a fast-track approval system that the country put in place in 2014. Designed to speed access to regenerative therapies, the law allows prospective treatments to be marketed and used as long as they have been proved to be safe. Only a suggestion of efficacy is required — with more-convincing data supposed to be gathered retrospectively from patients who have been given the approved treatment. The system has its critics — Nature among them (see Nature 528, 163–164; 2015). The latest move adds further concerns. The therapy is the work of a physician who was also the first to take advantage of the new law with a related treatment: Osaka University cardiac surgeon Yoshiki Sawa. There is no suggestion that Sawa has not followed the rules, set out by the Pharmaceuticals and Medical Devices Agency. He has. The issue is whether those rules are adequate and appropriate and have the welfare of patients at their heart. They do not. Treatments of no proven efficacy are being sold to patients (who effectively subsidize the clinical trials to test them). They receive no refund if the therapy is subsequently found not to work. Patients also take risks: they undergo immunosuppression and the surgery itself. The new study takes induced pluripotent stem cells (iPS cells) that have been banked and characterized to ensure they are safe, and converts them to heart-muscle cells. These are then spread into a thin sheet that is attached to the weakened heart muscle. It is only the second clinical application of iPS cells and is generating excitement around the world. The problem is that the earlier treatment from Sawa — which is ongoing under the fast-track system — has yet to produce convincing results. In that treatment, approved in September 2015, patients received a sheet of muscle cells made from their own leg tissue, rather than from iPS cells. Called HeartSheet, the muscle sheet is attached to weakened heart muscle that has usually been damaged as a result of a heart attack or plaque build-up and is often the cause of heart failure. The scientists behind the treatment speculate that the muscle cells work by releasing growth factors, not by becoming supporting tissue themselves. Other researchers are sceptical. Now there are two new treatments being investigated for the same condition, and it’s impossible to know yet whether either will work or which might be best for individual patients. It makes sense that heart-muscle cells (used in the second study) might work better for the heart than leg-muscle cells (used in the first). Indeed, it was reported a decade ago that injecting muscle cells from the leg did not improve heart function (P. Menasché et al. Circulation 17,1189–1200; 2008). Most physicians hoping to treat heart disease by way of regenerative medicine have moved on to other strategies, with many looking to heart-muscle cells. That doesn’t mean HeartSheet cannot work, but it does raise the question of whether patients who are given it will benefit. Sawa himself has raised the issue. At a symposium last month touting the new iPS cell trial, he said “leg cells are not good, well, at least not enough”. And the Osaka University web page announcing the iPS cell trial says that HeartSheet was found to be ineffective for more serious cases. Sawa told Nature that the cells work in some cases, but that he expects the new iPS cell therapy to be more effective. All this places a question mark over how the efficacy of HeartSheet can be proved as required. Half way through its scheduled 5-year plan, fewer than 10 patients — of the 60 required by the terms of its approval — have received the treatment. If the trial doesn’t make 60, the health ministry told Nature, there would either be an extension or the ministry would try to make a decision on the basis of the available data. Some physicians have called for the HeartSheet tests to end and the data to be assessed before the new iPS cell study can begin. That might be an over-reaction, but pressure on the Japanese government is increasing. The government needs to move quickly to make sure that evaluation of the HeartSheet therapy is as rigorous as promised. As more treatments emerge, officials should make sure that — fast track or not — they have a valid claim to efficacy before being sold to patients. A therapy for heart disease could be the first iPS-cell clinical breakthrough that Japan so ardently desires. The country shouldn’t sell short the promising technology or the patients who hope to benefit from it. Nature 557, 611-612 (2018) doi: 10.1038/d41586-018-05284-w |

 一键同布到我集网·各家微博
一键同布到我集网·各家微博