|
管理员

|
重磅!美国首例新冠病毒确诊病例康复全记录
2020年02月01日 07:22:55 来源:药明康德 重磅!美国首例新冠病毒确诊病例康复全记录 ▎药明康德/报道今日,权威医学期刊《新英格兰医学杂志》(NEJM)在线发表了多篇关于新型冠状病毒(2019-nCoV)病例的论文,其中一篇介绍了美国首例确诊病例的诊疗过程以及临床表现。 这些信息的公开,对于医务人员更好地了解疾病的特征,指导患者的诊疗有着重要的意义。 今天的这篇文章中,我们也将为各位读者朋友们介绍其中的一些要点。   患者背景介绍 据《新英格兰医学杂志》报道,这名患者是一位35岁的男性。今年1月15日,他结束了在武汉的探亲,返回美国。返美后的第一天,他就开始咳嗽。第二天,在咳嗽之余,他还自觉有一些发热。第三天,他选择在家休息,并依旧感觉有发热现象。而在了解到美国CDC的健康警报后,结合自己的症状和武汉旅行史,他决定去看医生。1月19日,也就是返美后的第四天,他前往了位于华盛顿州斯诺霍米什(Snohomish,位于西雅图北边约45分钟车程)郡的一家急诊室。值得一提的是,这名患者在挂号时,就已佩戴口罩,有着良好的保护意识。挂号后的约20分钟后,他被领入检查室接受医生的检查,他也同时向医生汇报了自己的旅行史。 本论文指出,患者汇报并没有前往华南海鲜市场,也没有和已知的病人进行过接触。初步诊断结果 在急诊室,医生们取得了关于这名患者的第一批生理数据。具体来看,当时这名患者的体温为37.2度,肺部有听诊音。然而,X光胸片的结果则没有异常。 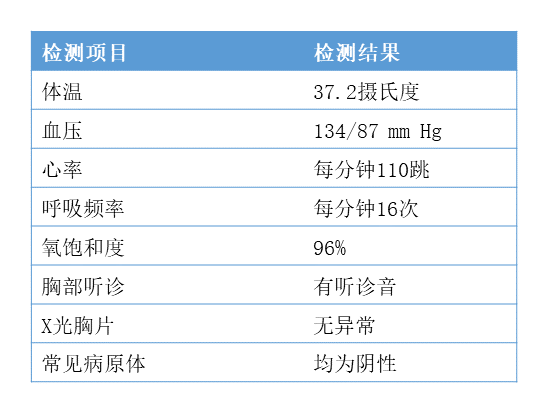 ▲该患者的初步诊断结果(数据来源 :参考资料[1];制图:药明康德内容团队 )医务人员也收集了他的鼻咽拭子样本,用于病原体的检验,而甲流和乙流的检验结果均为阴性。48小时后,剩余的检验结果也陆续返回。医生们同样没有检测出副流感、呼吸道合胞病毒、鼻病毒、以及腺病毒的存在。此外,医生们还检测了4种已知能感染人类,导致疾病的常见冠状病毒(HKU1,NL63,229E,OC43),结果仍然是阴性。 基于这些阴性结果,以及患者的武汉旅行史,美国CDC决定检测这名患者是否携带新型冠状病毒,并要求这名患者回家,在当地卫生部门的监督下进行自我隔离。 1月20日,检测结果返回:该患者的鼻咽拭子和口咽拭子样本,均呈现新型冠状病毒阳性。于是,这名患者被收治于西雅图附近一家医院的隔离病房进行治疗。住院后的早期治疗 在《新英格兰医学杂志》的这篇论文里,作者们提到,在患者住院时,已出现恶心和呕吐现象。然而除了恶心和呕吐,以及持续的发烧和干咳之外,这名患者并没有感到任何气短或胸痛,主要生理指标也在正常范围之内。因此,最初这名患者只是接受辅助性的照料,包括每天输注2升生理盐水,并接受恩丹西酮的治疗,以缓解恶心。在接下来的5天里,除了伴有心动过速的高烧之外,这名患者的主要生理指标依旧维持稳定。不过在住院后的第二天,他出现了腹泻和腹部不适。值得一提的是,在腹泻的粪便样本中,医生们也检测出了新型冠状病毒的存在(rRT-PCR结果阳性)。 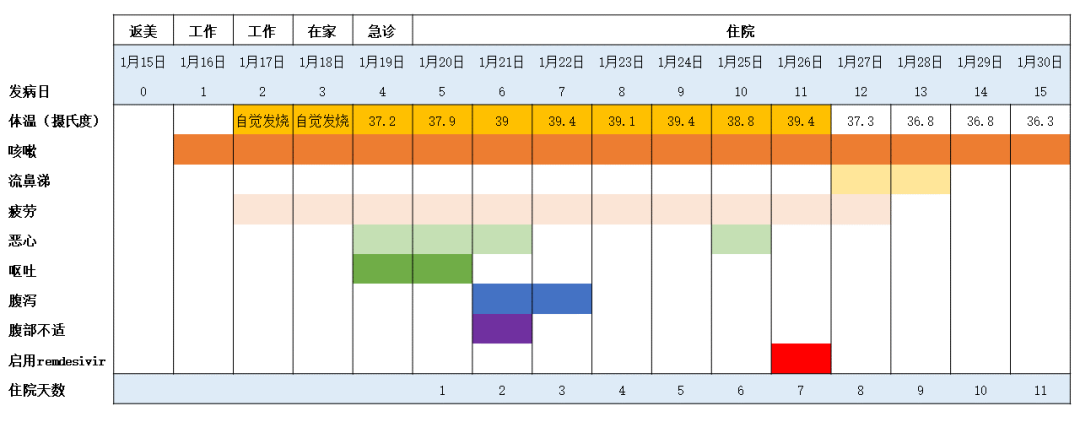 ▲该患者的症状全记录,启用remdesivir治疗的日期也做了注明(数据来源 :参考资料[1];制图:药明康德内容团队;点击图片可以观看大图 ) ▲该患者的症状全记录,启用remdesivir治疗的日期也做了注明(数据来源 :参考资料[1];制图:药明康德内容团队;点击图片可以观看大图 )但总体来说,在住院的前几天,治疗依旧为辅助性为主,以协助患者控制症状。病情的恶化与治疗 本篇论文的作者们汇报说,在住院后的第三天,这名患者的X光胸片看起来依旧没有异常。然而到了住院的第五天,在左肺下叶出现了肺炎的特征。在出现肺炎特征的同一天晚上,患者的呼吸情况也有所变化,氧饱和度下降到了90%。第六天,医生们决定为他输氧。考虑到病情的恶化,以及担心患者出现获得性肺炎,医生们开始使用万古霉素和头孢吡肟两种抗生素进行治疗。 而在住院的第六天,这名患者的X光胸片结果显示出非典型性肺炎的特征。基于患者的胸片结果,以及持续高烧,需要吸氧,且多个部位的样本出现新型冠状病毒的阳性结果,医生们决定为其提供一种尚未获批的药物——由吉利德(Gilead)公司研发的抗病毒药remdesivir。这是一种核苷酸类似物前药,能够抑制依赖RNA的RNA合成酶(RdRp)。原先,这种在研疗法计划用于埃博拉病毒治疗,但冠状病毒里同样有RdRp。因此,这种在研疗法也有望对冠状病毒进行抑制。 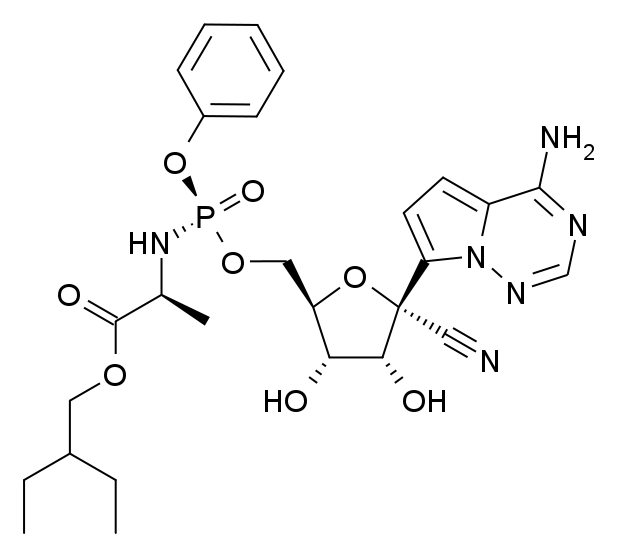 ▲这名患者在接受了remdesivir的治疗后,症状出现缓解。图为remdesivir的结构式(图片来源:Meodipt [Public domain] )在住院的第七天晚上,这名患者接受了remdesivir的静脉输注。第八天,这名患者的临床症状出现了立竿见影的改善。他不再需要吸氧,氧饱和度也恢复到了94%-96%。除了干咳和流鼻涕外,他已没有其他症状。 本论文发表于美国时间1月31日。在1月30日,这名患者虽仍在住院中,但已退烧。唯一的症状就是咳嗽,且严重程度与日俱减。结语 在论文的讨论环节,作者们指出在这名患者生病(并非住院)的第4天和第7天,病毒具有很高的载量水平,因此有传播的潜力。而且粪便检测出现新型冠状病毒阳性,也值得注意。对于在呼吸道外的潜在影响,目前我们还不甚了解。而从这一个病例来看,早期他只有发烧和咳嗽的现象,直到症状出现的第九天,病情才进展到肺炎的阶段。考虑到早期的症状非常轻微,且和其他冬季的传染性疾病有着类似之处,这也增加了诊断的难度。 关于remdesivir的治疗,作者们认为这是基于患者恶化的病情,使用美国的“同情用药”(compassionate use)原则进行的治疗。尽管在治疗之后,这名患者的病情出现了迅速的缓解,但我们依然需要进行随机对照的临床试验,来确定remdesivir和其他在研药物在治疗新型冠状病毒感染上的安全性和有效性。我们也期待更多临床数据可以得到公布和发表,让知识武装我们的大脑,用临床上的成功病例指导治疗,早日让患者摆脱疾病之苦! 本文题图来自Pixabay。 参考资料: [1] Michelle L. Holshue et al., (2020), First Case of 2019 Novel Coronavirus in the United States, NEJM, DOI: 10.1056/NEJMoa2001191 注:本文旨在介绍医药健康研究进展,不是治疗方案推荐。如需获得治疗方案指导,请前往正规医院就诊。 First Case of 2019 Novel Coronavirus in the United States https://www.nejm.org/doi/full/10.1056/NEJMoa2001191 First U.S. Confirmed Case of 2019-nCoV InfectionM.L. Holshue and Others A healthy 35-year-old man who had visited Wuhan, China, presented with cough and fever that progressed to pneumonia. This report describes the diagnosis, clinical course, and management of the condition. The case highlights the importance of close coordination between clinicians and public health authorities at the local, state, and federal levels. Summary An outbreak of novel coronavirus (2019-nCoV) that began in Wuhan, China, has spread rapidly, with cases now confirmed in multiple countries. We report the first case of 2019-nCoV infection confirmed in the United States and describe the identification, diagnosis, clinical course, and management of the case, including the patient’s initial mild symptoms at presentation with progression to pneumonia on day 9 of illness. This case highlights the importance of close coordination between clinicians and public health authorities at the local, state, and federal levels, as well as the need for rapid dissemination of clinical information related to the care of patients with this emerging infection.[/p][p] On December 31, 2019, China reported a cluster of cases of pneumonia in people associated with the Huanan Seafood Wholesale Market in Wuhan, Hubei Province.1On January 7, 2020, Chinese health authorities confirmed that this cluster was associated with a novel coronavirus, 2019-nCoV.2 Although cases were originally reported to be associated with exposure to the seafood market in Wuhan, current epidemiologic data indicate that person-to-person transmission of 2019-nCoV is occurring.3-6 As of January 30, 2020, a total of 9976 cases had been reported in at least 21 countries,7 including the first confirmed case of 2019-nCoV infection in the United States, reported on January 20, 2020. Investigations are under way worldwide to better understand transmission dynamics and the spectrum of clinical illness. This report describes the epidemiologic and clinical features of the first case of 2019-nCoV infection confirmed in the United States.[/p] Methods SPECIMEN COLLECTION Clinical specimens for 2019-nCoV diagnostic testing were obtained in accordance with CDC guidelines.12 Nasopharyngeal and oropharyngeal swab specimens were collected with synthetic fiber swabs; each swab was inserted into a separate sterile tube containing 2 to 3 ml of viral transport medium. Serum was collected in a serum separator tube and then centrifuged in accordance with CDC guidelines. The urine and stool specimens were each collected in sterile specimen containers. Specimens were stored between 2°C and 8°C until ready for shipment to the CDC. Specimens for repeat 2019-nCoV testing were collected on illness days 7, 11, and 12 and included nasopharyngeal and oropharyngeal swabs, serum, and urine and stool samples. DIAGNOSTIC TESTING FOR 2019-NCOV Clinical specimens were tested with an rRT-PCR assay that was developed from the publicly released virus sequence. Similar to previous diagnostic assays for severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), it has three nucleocapsid gene targets and a positive control target. A description of this assay13 and sequence information for the rRT-PCR panel primers and probes14 are available on the CDC Laboratory Information website for 2019-nCoV.15 GENETIC SEQUENCING On January 7, 2020, Chinese researchers shared the full genetic sequence of 2019-nCoV through the National Institutes of Health GenBank database16 and the Global Initiative on Sharing All Influenza Data (GISAID)17 database; a report about the isolation of 2019-nCoV was later published.18 Nucleic acid was extracted from rRT-PCR–positive specimens (oropharyngeal and nasopharyngeal) and used for whole-genome sequencing on both Sanger and next-generation sequencing platforms (Illumina and MinIon). Sequence assembly was completed with the use of Sequencher software, version 5.4.6 (Sanger); minimap software, version 2.17 (MinIon); and freebayes software, version 1.3.1 (MiSeq). Complete genomes were compared with the available 2019-nCoV reference sequence (GenBank accession number NC_045512.2).  来源:药明康德 重磅!美国首例新冠病毒确诊病例康复全记录 https://mp.weixin.qq.com/s/X2dO8dHcK3hYBYQ854R5Qw http://news.ifeng.com/c/7thfobDOVxe |
|
论坛版主

|
板凳#
发布于:2020-02-02 07:20
|
|
管理员

|
地板#
发布于:2020-02-02 13:40
|
|
管理员

|
4楼#
发布于:2020-02-02 14:08
|

 一键同布到我集网·各家微博
一键同布到我集网·各家微博